INNOVATION
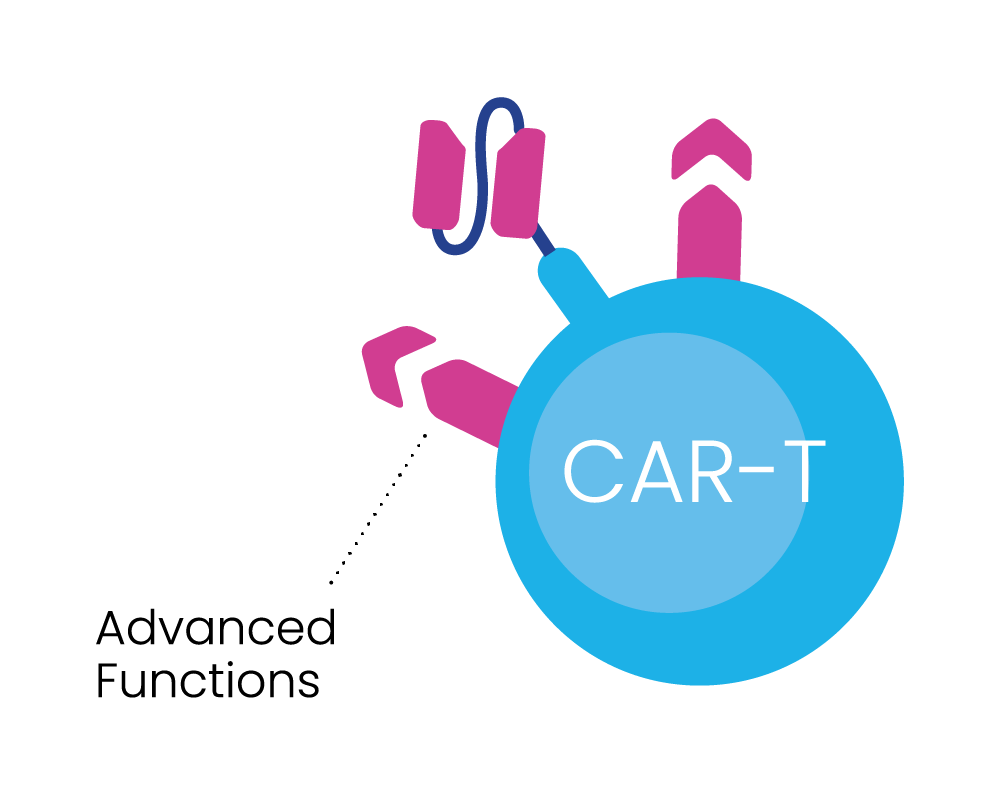
Inspired and ambitious, our researchers are pioneers in cancer therapeutic development – focussed on developing our BRiDGECAR™ system, which combines our nfP2X7-targeted technology with highly potent chimeric antigen receptor (CAR) T-cell therapy and multiple targeted antibodies.
If successful, we will be one step closer to our vision – targeted treatment for millions of cancer patients worldwide.
THE BRiDGECAR™ SYSTEM
WHY BRiDGECAR™?
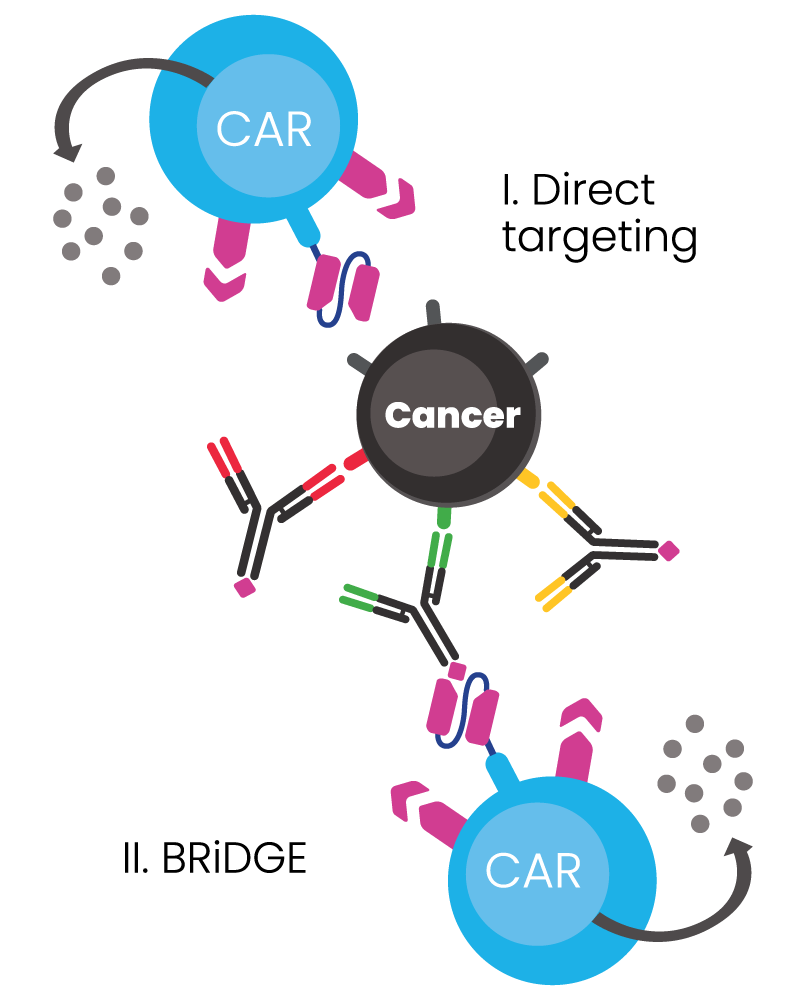
BRiDGECAR™ has an advantage over competitors due to its dual functional capability:
- It has a direct targeting function via nfP2X7 derived antibody sequences, and
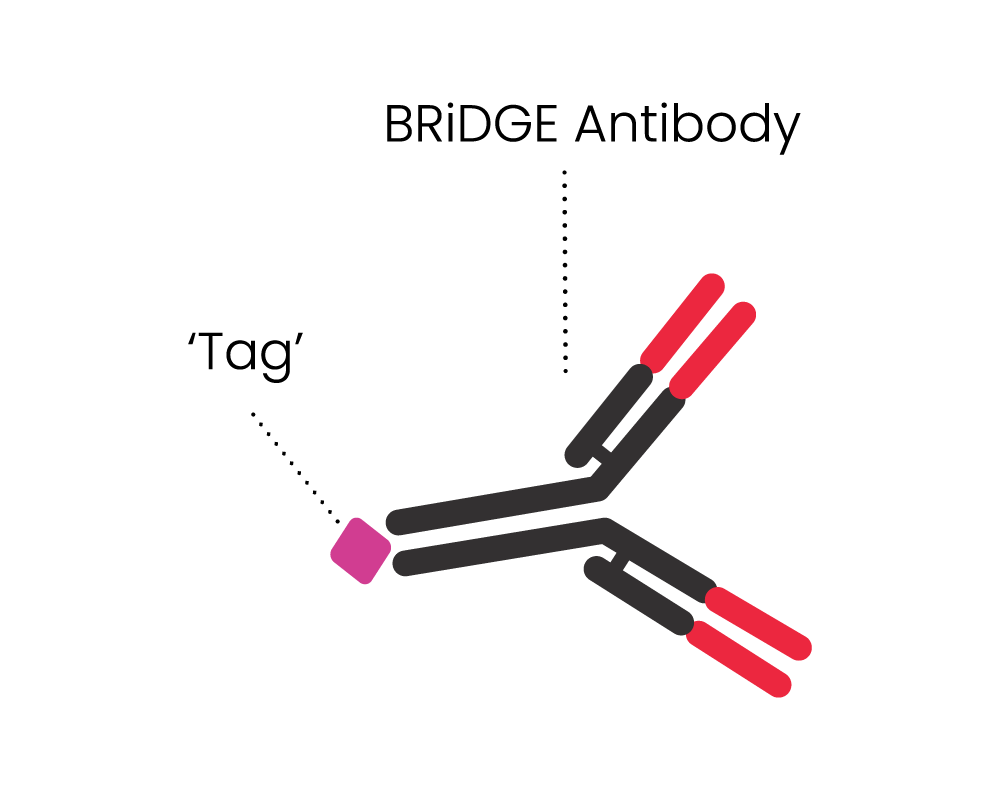
- It can attack cancer via additional BRiDGE antibodies, enhancing the cancer cell killing and minimising treatment resistance and patient relapse
BRiDGECAR™ incorporates industry leading advanced molecules that boost long-term cancer cell killing.
BRiDGECAR™ has the potential to treat most types of cancer.
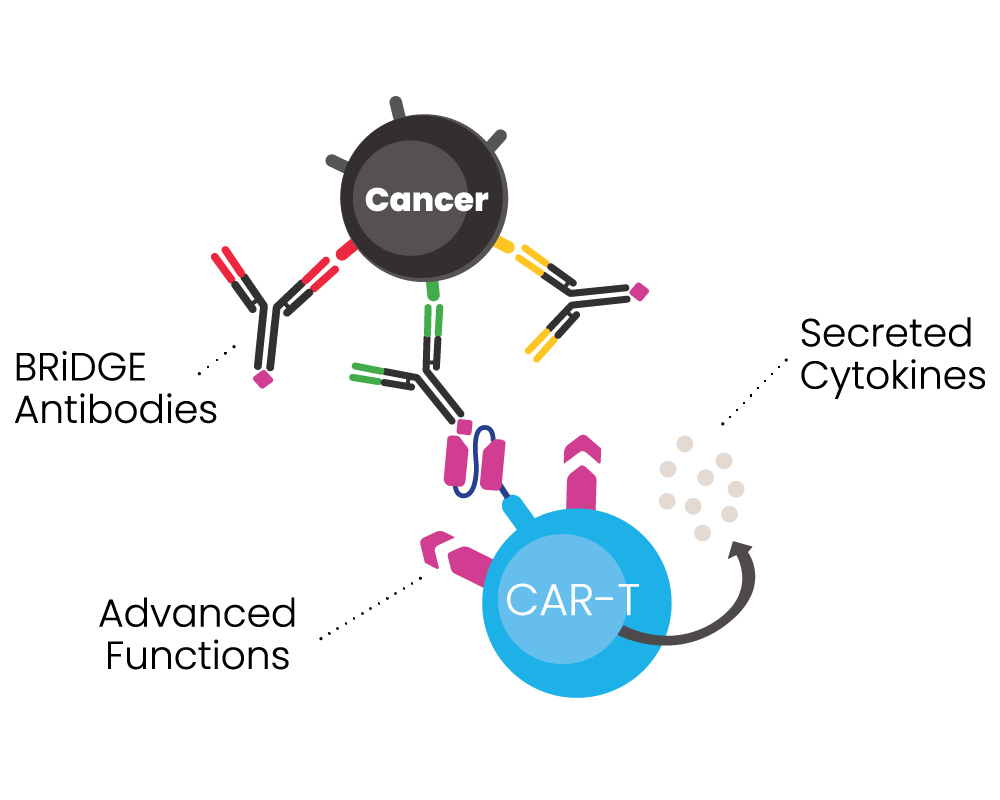
BIOSCEPTRE’S BRiDGECAR™ DUAL-FUNCTIONALITY
The BRiDGECAR™ targets cancer both directly and via the BRiDGE™ antibodies.
BRiDGECAR™ T cells persist, hunting down and killing cancer cells between BRiDGE antibody treatments.
BRiDGECAR™ may provide ongoing tumour suppression via nfP2X7 after the administration of the BRiDGE antibodies has ceased.
Competitor approaches cannot do this.
TARGETED ANTIBODY THERAPY
We are also developing antibodies that specifically target nfP2X7 for use in both solid and haematological tumours. Our preclinical data is very promising and studies show an excellent safety profile. By exploiting the modular nature of the antibody structure, the same panel of antibody sequences can be used to generate a variety of therapeutic antibody formats including, but not limited to, monoclonal, bispecifics, antibody-drug conjugates (ADCs), and bispecific T-cell engagers (BiTEs) in addition to our CAR-T program.