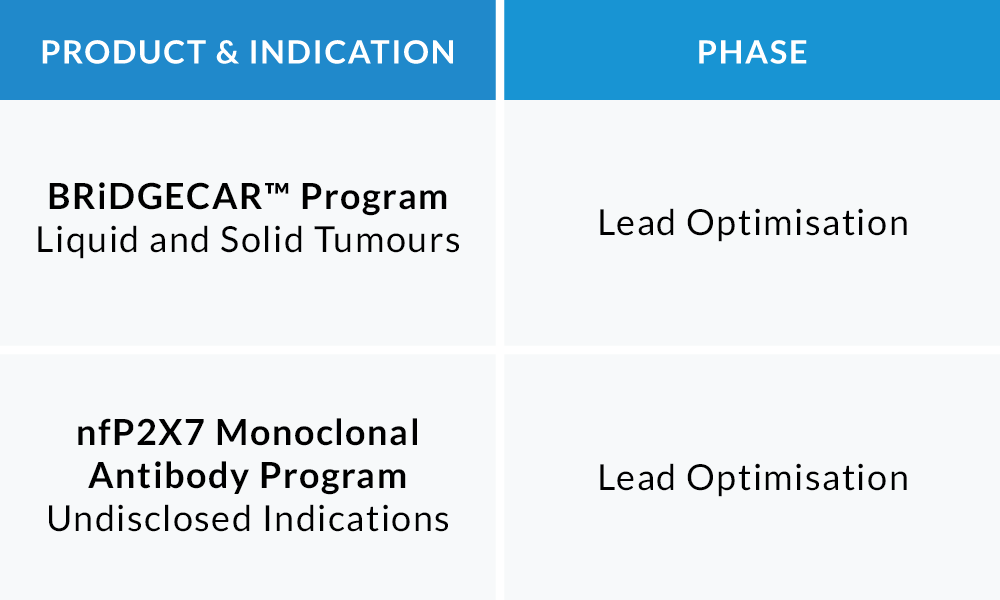
PIPELINE
We know that our ground-breaking treatments have the potential to treat a range of malignancies and make a drastic difference to lives of millions of cancer patients, globally.
There are unique synergies when CAR T therapeutic technology is applied to the novel oncology target nfP2X7 that has been characterised and developed by Biosceptre.
Our pioneering BRiDGECAR™ system combines highly potent CAR-T therapy with multiple targeted antibodies and is designed to be the next generation of cancer therapy, with the potential of delivering unprecedented outcomes to many types of cancer.